Seznamy Atom Definition Chemistry Čerstvý
Seznamy Atom Definition Chemistry Čerstvý. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.
Nejlepší Chemistry I Atoms And Molecules
Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.
Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.

Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom... The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity... Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.

Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). . Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.
Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity... The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms... Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence... Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence... The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity... Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.

Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom... Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion... Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom... Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.. .. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion... The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another... Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity... Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion... Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. .. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another... Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another... Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.
The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms... Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom... Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen)... Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom... Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion... Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.. .. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.

Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen)... Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms... Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.

Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom... Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence... Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence... Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.

Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.

Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.

Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.

Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).. .. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. . The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom... Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. .. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. . Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another... Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms... Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.

Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence... . Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.

Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.. .. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.

Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.

Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom... Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.

Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms.. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom... Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.
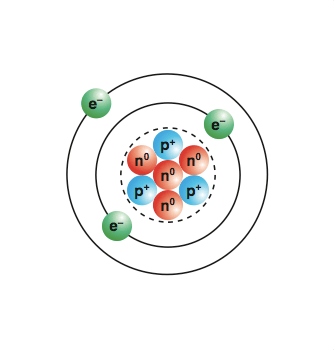
Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.

Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom.
The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence.. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen). Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Sep 22, 2021 · in chemistry, a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Energy is supplied for removing an atom implies that energy will be released if an extra electron is added to the atom.. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity.

Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Jan 24, 2019 · the iupac formal definition of valence is the maximum number of univalent atoms that may combine with an atom. The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Usually, the definition is based on the maximum number of either hydrogen atom or chlorine atoms. Note the iupac only defines a single valence value (the maximum), while atoms are known to be capable of displaying more than one valence. Nov 14, 2017 · hydrogen bond definition hydrogen bond is an attractive force between a partially positive charged hydrogen and a partially negative charged atom (oxygen and nitrogen).
