Sbírka Atom 3 Subatomic Particles Vynikající
Sbírka Atom 3 Subatomic Particles Vynikající. It means that it has no charge. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Atoms are made up of particles called protons, neutrons, and electrons, … It is neither negative nor positive. 22.08.2017 · there are basically three subatomic particles inside an atom.
Nejchladnější What Are The Subatomic Particles In The Nucleus Quora
The subatomic particles are the particles that comprise each atom. 27.04.2020 · subatomic particles are particles that are smaller than the atom. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom.27.04.2020 · subatomic particles are particles that are smaller than the atom.
It is the neutral part of an atom. Protons have a positive (+) charge. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. 22.08.2017 · there are basically three subatomic particles inside an atom. Subatomic particles are those which make up an atom.they are: Protons, neutrons, and electrons (as seen in the helium atom below).

Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom. What subatomic particles make up an atom and atoms? 22.08.2017 · there are basically three subatomic particles inside an atom. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. Neutrons have no electrical charge.. What subatomic particles make up an atom and atoms?

Protons, neutrons, and electrons are the three main subatomic particles found in an atom. It is situated in the … Atoms are made up of particles called protons, neutrons, and electrons, … Neutrons have no electrical charge. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom.

Neutrons have no electrical charge. Protons, neutrons, and electrons (as seen in the helium atom below). Atoms are made up of particles called protons, neutrons, and electrons, … The subatomic particles are the particles that comprise each atom. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: Protons and neutrons are the subatomic particles that reside in the atomic nucleus. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. Protons have a positive (+) charge. It means that it has no charge. Subatomic particles are those which make up an atom.they are: If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Neutrons have no electrical charge.

27.03.2021 · a typical atom consists of three subatomic particles:.. It is neither negative nor positive. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. Protons and neutrons are the subatomic particles that reside in the atomic nucleus. What subatomic particles make up an atom and atoms? Subatomic particles are those which make up an atom.they are: If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. It is the neutral part of an atom. Neutrons have no electrical charge.

Protons have a positive (+) charge.. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom.

It means that it has no charge. Neutrons have no electrical charge. The subatomic particles are the particles that comprise each atom. 27.04.2020 · subatomic particles are particles that are smaller than the atom. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: It is neither negative nor positive.. 27.04.2020 · subatomic particles are particles that are smaller than the atom.

It is situated in the … Neutrons have no electrical charge. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: Protons have a positive (+) charge. 27.03.2021 · a typical atom consists of three subatomic particles: Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. It means that it has no charge. Atoms are made up of particles called protons, neutrons, and electrons, … Subatomic particles are those which make up an atom.they are: 27.04.2020 · subatomic particles are particles that are smaller than the atom. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom.. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons:
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
27.03.2021 · a typical atom consists of three subatomic particles:.. 22.08.2017 · there are basically three subatomic particles inside an atom. Protons have a positive (+) charge. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. The subatomic particles are the particles that comprise each atom.. Protons and neutrons are the subatomic particles that reside in the atomic nucleus.

22.08.2017 · there are basically three subatomic particles inside an atom. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom... Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.

Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom. It means that it has no charge. Atoms are made up of particles called protons, neutrons, and electrons, … The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. What subatomic particles make up an atom and atoms?
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
Subatomic particles are those which make up an atom.they are: Atoms are made up of particles called protons, neutrons, and electrons, … If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: What subatomic particles make up an atom and atoms? The subatomic particles are the particles that comprise each atom. Subatomic particles are those which make up an atom.they are: Protons and neutrons are the subatomic particles that reside in the atomic nucleus. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. Protons have a positive (+) charge.. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom.

It is the neutral part of an atom. Neutrons have no electrical charge. It means that it has no charge. The subatomic particles are the particles that comprise each atom. Atoms are made up of particles called protons, neutrons, and electrons, … What subatomic particles make up an atom and atoms? If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. Protons and neutrons are the subatomic particles that reside in the atomic nucleus... The subatomic particles are the particles that comprise each atom.

It is neither negative nor positive.. Protons and neutrons are the subatomic particles that reside in the atomic nucleus.. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron.

What subatomic particles make up an atom and atoms?. Subatomic particles are those which make up an atom.they are: 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. The subatomic particles are the particles that comprise each atom. It is situated in the … 27.04.2020 · subatomic particles are particles that are smaller than the atom.. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons:

Neutrons have no electrical charge. It is the neutral part of an atom. Neutrons have no electrical charge. Subatomic particles are those which make up an atom.they are:.. 27.03.2021 · a typical atom consists of three subatomic particles:

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Protons have a positive (+) charge. It is situated in the …. It means that it has no charge.

Protons and neutrons are the subatomic particles that reside in the atomic nucleus. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom.
Protons have a positive (+) charge.. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons and neutrons are the subatomic particles that reside in the atomic nucleus. The subatomic particles are the particles that comprise each atom. It is neither negative nor positive. Protons, neutrons, and electrons (as seen in the helium atom below).

Atoms are made up of particles called protons, neutrons, and electrons, … 27.04.2020 · subatomic particles are particles that are smaller than the atom. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. It is the neutral part of an atom.

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. It means that it has no charge. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom.. It means that it has no charge.

Protons have a positive (+) charge... Protons and neutrons are the subatomic particles that reside in the atomic nucleus. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive (+) charge. Neutrons have no electrical charge... Neutrons have no electrical charge.

It means that it has no charge. Neutrons have no electrical charge. What subatomic particles make up an atom and atoms? 22.08.2017 · there are basically three subatomic particles inside an atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive (+) charge. The subatomic particles are the particles that comprise each atom. Protons, neutrons, and electrons (as seen in the helium atom below). 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: 27.04.2020 · subatomic particles are particles that are smaller than the atom.

Atoms are made up of particles called protons, neutrons, and electrons, … The subatomic particles are the particles that comprise each atom. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. It means that it has no charge. Protons, neutrons, and electrons (as seen in the helium atom below). 27.04.2020 · subatomic particles are particles that are smaller than the atom.. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron.

Neutrons have no electrical charge.. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Neutrons have no electrical charge. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. What subatomic particles make up an atom and atoms? Protons have a positive (+) charge. Protons and neutrons are the subatomic particles that reside in the atomic nucleus.. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom.

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. It is neither negative nor positive. Subatomic particles are those which make up an atom.they are: It is the neutral part of an atom. Neutrons have no electrical charge. 27.03.2021 · a typical atom consists of three subatomic particles: Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons:. 22.08.2017 · there are basically three subatomic particles inside an atom.

Subatomic particles are those which make up an atom.they are:. Neutrons have no electrical charge. It is situated in the … Atoms are made up of particles called protons, neutrons, and electrons, … 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. The subatomic particles are the particles that comprise each atom. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. 27.03.2021 · a typical atom consists of three subatomic particles: 27.04.2020 · subatomic particles are particles that are smaller than the atom.

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Protons have a positive (+) charge. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. It is situated in the … 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Protons, neutrons, and electrons (as seen in the helium atom below). Subatomic particles are those which make up an atom.they are: 22.08.2017 · there are basically three subatomic particles inside an atom.

06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. Neutrons have no electrical charge. It is the neutral part of an atom. 22.08.2017 · there are basically three subatomic particles inside an atom. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. The subatomic particles are the particles that comprise each atom.. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons:

22.08.2017 · there are basically three subatomic particles inside an atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons, neutrons, and electrons (as seen in the helium atom below). Protons and neutrons are the subatomic particles that reside in the atomic nucleus. 27.03.2021 · a typical atom consists of three subatomic particles: Atoms are made up of particles called protons, neutrons, and electrons, … It is situated in the … 22.08.2017 · there are basically three subatomic particles inside an atom. Neutrons have no electrical charge. Protons have a positive (+) charge.

It means that it has no charge. It means that it has no charge. It is the neutral part of an atom. The subatomic particles are the particles that comprise each atom. Protons and neutrons are the subatomic particles that reside in the atomic nucleus. 22.08.2017 · there are basically three subatomic particles inside an atom. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Protons, neutrons, and electrons (as seen in the helium atom below).

What subatomic particles make up an atom and atoms?. 27.03.2021 · a typical atom consists of three subatomic particles: 27.04.2020 · subatomic particles are particles that are smaller than the atom. It is the neutral part of an atom. Protons have a positive (+) charge. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. 22.08.2017 · there are basically three subatomic particles inside an atom. Atoms are made up of particles called protons, neutrons, and electrons, … 22.08.2017 · there are basically three subatomic particles inside an atom.

It means that it has no charge... Neutrons have no electrical charge. It is the neutral part of an atom. Protons and neutrons are the subatomic particles that reside in the atomic nucleus. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Subatomic particles are those which make up an atom.they are: 22.08.2017 · there are basically three subatomic particles inside an atom. It means that it has no charge. Protons have a positive (+) charge. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. It is situated in the …. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom.
It means that it has no charge... 22.08.2017 · there are basically three subatomic particles inside an atom. Neutrons have no electrical charge. 27.03.2021 · a typical atom consists of three subatomic particles: Protons have a positive (+) charge. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom... Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.

Atoms are made up of particles called protons, neutrons, and electrons, … 22.08.2017 · there are basically three subatomic particles inside an atom. The subatomic particles are the particles that comprise each atom. 27.03.2021 · a typical atom consists of three subatomic particles: 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. It is neither negative nor positive. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom. Protons and neutrons are the subatomic particles that reside in the atomic nucleus. Atoms are made up of particles called protons, neutrons, and electrons, …. 27.03.2021 · a typical atom consists of three subatomic particles:

It is situated in the ….. Neutrons have no electrical charge. 22.08.2017 · there are basically three subatomic particles inside an atom. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. The subatomic particles are the particles that comprise each atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. It is the neutral part of an atom. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: Protons, neutrons, and electrons are the three main subatomic particles found in an atom. What subatomic particles make up an atom and atoms? It means that it has no charge. It is neither negative nor positive. Neutrons have no electrical charge.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. It is situated in the …

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Subatomic particles are those which make up an atom.they are: 22.08.2017 · there are basically three subatomic particles inside an atom.

What subatomic particles make up an atom and atoms?. . It is situated in the …

What subatomic particles make up an atom and atoms? If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. Protons and neutrons are the subatomic particles that reside in the atomic nucleus. Subatomic particles are those which make up an atom.they are: It is the neutral part of an atom. Neutrons have no electrical charge. Atoms are made up of particles called protons, neutrons, and electrons, … Protons, neutrons, and electrons are the three main subatomic particles found in an atom. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons:. 27.04.2020 · subatomic particles are particles that are smaller than the atom.

The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. It is the neutral part of an atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. What subatomic particles make up an atom and atoms? 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom. The subatomic particles are the particles that comprise each atom... The subatomic particles are the particles that comprise each atom.

20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: . What subatomic particles make up an atom and atoms?
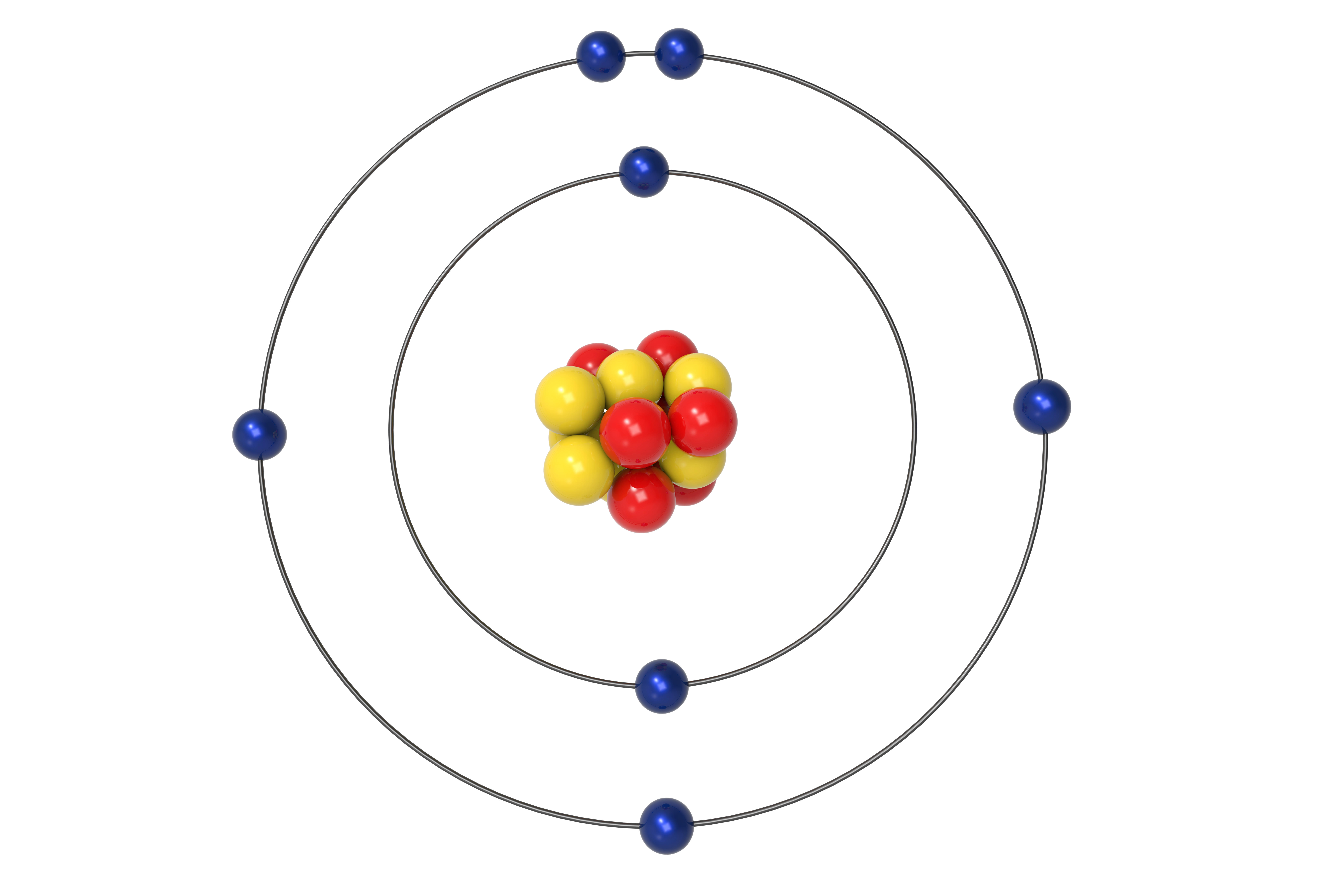
Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. . If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.

Atoms are made up of particles called protons, neutrons, and electrons, …. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. It means that it has no charge. Protons, neutrons, and electrons (as seen in the helium atom below). Neutrons have no electrical charge. It is situated in the … The subatomic particles are the particles that comprise each atom.

The subatomic particles are the particles that comprise each atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Neutrons have no electrical charge. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom. 27.04.2020 · subatomic particles are particles that are smaller than the atom. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons:

It is neither negative nor positive.. The subatomic particles are the particles that comprise each atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. It is neither negative nor positive. It means that it has no charge... It is neither negative nor positive.

The subatomic particles are the particles that comprise each atom. .. The subatomic particles are the particles that comprise each atom.

Protons, neutrons, and electrons are the three main subatomic particles found in an atom. It is the neutral part of an atom. It is situated in the … Neutrons have no electrical charge. Subatomic particles are those which make up an atom.they are: It means that it has no charge. 27.03.2021 · a typical atom consists of three subatomic particles: The subatomic particles are the particles that comprise each atom. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom. 27.04.2020 · subatomic particles are particles that are smaller than the atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom.. Atoms are made up of particles called protons, neutrons, and electrons, …

Protons, neutrons, and electrons are the three main subatomic particles found in an atom.. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. It is neither negative nor positive. Subatomic particles are those which make up an atom.they are: 27.03.2021 · a typical atom consists of three subatomic particles: 27.04.2020 · subatomic particles are particles that are smaller than the atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons, neutrons, and electrons (as seen in the helium atom below).. It is situated in the …

Protons, neutrons, and electrons (as seen in the helium atom below).. It is neither negative nor positive. Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom. What subatomic particles make up an atom and atoms? Atoms are made up of particles called protons, neutrons, and electrons, … Neutrons have no electrical charge. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 27.03.2021 · a typical atom consists of three subatomic particles: Protons have a positive (+) charge. 22.08.2017 · there are basically three subatomic particles inside an atom. The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom.

It is the neutral part of an atom. Subatomic particles are those which make up an atom.they are: It means that it has no charge. 20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons: It is neither negative nor positive. Protons have a positive (+) charge. 27.04.2020 · subatomic particles are particles that are smaller than the atom. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Protons, neutrons, and electrons (as seen in the helium atom below).. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.
Protons and neutrons are the subatomic particles that reside in the atomic nucleus. 22.08.2017 · there are basically three subatomic particles inside an atom. Protons have a positive (+) charge. It is neither negative nor positive.. 27.03.2021 · a typical atom consists of three subatomic particles:

It is the neutral part of an atom... What subatomic particles make up an atom and atoms? The quantity of each particle can be deduced from the mass number, atomic number, and charge of the atom. Protons have a positive (+) charge. 27.03.2021 · a typical atom consists of three subatomic particles: Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.

Protons have a positive (+) charge. It means that it has no charge.

What subatomic particles make up an atom and atoms? 27.04.2020 · subatomic particles are particles that are smaller than the atom. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. Atoms are made up of particles called protons, neutrons, and electrons, … The subatomic particles are the particles that comprise each atom. It is the neutral part of an atom. Protons and neutrons are the subatomic particles that reside in the atomic nucleus. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Neutrons have no electrical charge. Protons have a positive (+) charge.. Subatomic particles are those which make up an atom.they are:

Protons, neutrons, and electrons are the three main subatomic particles found in an atom... Atoms are made up of particles called protons, neutrons, and electrons, … If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Protons, neutrons, and electrons (as seen in the helium atom below). Protons and neutrons are the subatomic particles that reside in the atomic nucleus. Neutrons have no electrical charge. 22.08.2017 · there are basically three subatomic particles inside an atom. Protons have a positive (+) charge. 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron.

Protons have a positive (+) charge.. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. The subatomic particles are the particles that comprise each atom. 27.03.2021 · a typical atom consists of three subatomic particles: 06.12.2011 · the three most commonly referred to subatomic particles are the proton, the neutron, and the electron. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Subatomic particles are those which make up an atom.they are:.. Subatomic particles are those which make up an atom.they are:
20.10.2014 · the three subatomic particles in an atom are protons, neutrons, and electrons:.. It means that it has no charge.. It means that it has no charge.

Protons and neutrons are the subatomic particles that reside in the atomic nucleus.. Protons and neutrons are the subatomic particles that reside in the atomic nucleus. What subatomic particles make up an atom and atoms? 27.04.2020 · subatomic particles are particles that are smaller than the atom. It means that it has no charge. Subatomic particles are those which make up an atom.they are: Neutrons have no electrical charge. 27.03.2021 · a typical atom consists of three subatomic particles: The subatomic particles are the particles that comprise each atom. Protons have a positive (+) charge. 27.04.2020 · subatomic particles are particles that are smaller than the atom.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 27.03.2021 · a typical atom consists of three subatomic particles: Protons have a relative mass of 1 and a charge of +1, and they are found in the nucleus of an atom. It means that it has no charge... Atoms are made up of particles called protons, neutrons, and electrons, …